Background: Understanding the treatment experience of patients (pts) with relapsed/refractory multiple myeloma (RRMM) is important to providing timely, appropriate supportive care. A multi-center trial (NCT05053607) is examining patient experience with isatuximab for treatment of RRMM, triangulating quantitative data from patient-reported outcomes (PRO) and wearable devices, along with qualitative data collected via individual interviews. Here we present outcomes from the qualitative data analysis, the aim of which was to describe experience with diagnosis and treatment, as well as the digital life coaching (DLC) intervention.
Study Design and Methods: In a prospective, quasi-experimental study, 50 individuals aged ≥18 years are consented and enrolled in a 3-month DLC intervention. PROs are collected at baseline and up to once per month for 3 months in addition to physical activity data collected via wrist-worn activity tracker. Up to 10 participants were purposefully selected to participate in an individual interview of up to 1 hour at one time point between weeks 8-12 of study participation. Qualitative descriptive analysis was used to analyze study data and summarize key themes.
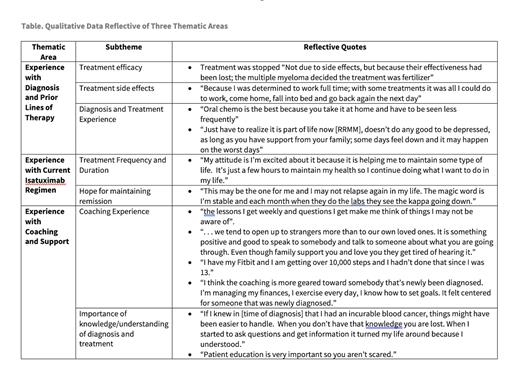
Results: A total of 8 individuals participated in qualitative interviews (2 men and 6 women). One identified as African American, 1 as Hispanic, and 6 as White. The mean age of participants was 67.6 (R= 47-86); average time since diagnosis was 8.25 (R=1-15); and average lines of therapy was 4.25 (R= 3-8). Responses were organized into three themes, focused on experience with: diagnosis and prior lines of therapy; current isatuximab inclusive therapy; and DLC, with highlighted quotes represented in the Table.
Participants self-reported the most common symptoms of prior lines of therapy were hair loss, gastrointestinal symptoms (e.g., nausea, reflux, upset stomach), and “steroid-related” symptoms of food cravings and insomnia. Previous lines of therapy were discontinued due to either treatment toxicity or disease progression; the most commonly reported treatment toxicities were blood clots, neuropathy, dizziness, and immunosuppression with associated infections or pneumonia.
Related specifically to their isatuximab-treatment regimens, most participants reported feeling a “burst of energy” but few to no side effects at the end of treatment. Several commented on the time commitment of having to go to the cancer center for infusions. Most stated the frequency and duration is acceptable given the outcomes of treatment. Insights into the measures of success of treatment ranged from how individuals felt while receiving therapy, to what degree therapy interfered with their ability to participate in activities of daily living, and the effect of treatment on their RRMM status. Several participants specifically spoke by name to the response of their Kappa free light chain test results being significantly lower.
When asked about what they found most supportive during treatment, knowledge was the most common response. They also reported family, having a plan, and spirituality/faith as helpful. Regarding their coaching experience on study all described it as “positive,” however, two reported that the content felt fundamental to them since they had been managing their diagnosis for several years. Benefits included having someone, particularly other than a family member, to speak to about their health, increased attention on healthy behaviors such as nutrition and exercise, and enhancing access to information and education about their diagnosis, treatment, and management.
Conclusions: Qualitative interviews among individuals with RRMM provided insight into the challenges but also hope associated with chronic management of their condition. Treatment with isatuximab regimens was perceived as logistically manageable with few side effects. Though some individuals felt their long-term experience with MM made the coaching less impactful, coaching was generally perceived as a positive experience, with many individuals self-reporting benefits related to health behaviors and outcomes.
Disclosures
Brassil:mConsulting: Consultancy; Pack Health, A Quest Diagnostics Company: Current Employment, Current holder of stock options in a privately-held company; Daiichi Sankyo: Research Funding; GSK: Research Funding; Sanofi: Research Funding; Gilead: Research Funding. Banerjee:Caribou: Consultancy; Pfizer: Consultancy; Pack Health: Research Funding; BMS: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Sanofi: Consultancy; SparkCures: Consultancy. Barr:Pack Health, A Quest Diagnostics Company: Current Employment. Cowan:Abbvie: Consultancy, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy; BMS: Consultancy, Research Funding; EUSA: Consultancy; GSK: Consultancy; Harpoon: Research Funding; Janssen: Consultancy, Research Funding; Nektar: Research Funding; Sanofi: Research Funding; Secura Bio: Consultancy. Manasanch:A: Consultancy; G: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal